Research
Neurotransmission relies on the fusion of synaptic vesicles (SV) with the presynaptic plasma membrane (exocytosis) to release neurotransmitters. After exocytosis, excess plasma membrane resulting from the addition of SV membrane is rapidly internalized by compensatory endocytosis and used to generate new SVs. Importantly, neuronal cells rely on these conserved endocytic and synaptic vesicle recycling processes to sustain high rates of activity. Animal models of defective endocytosis show accumulation of recycling intermediates at their synapses and prominent neurodegeneration and/or early lethality.
Although much knowledge has been gathered on the main proteins and lipids involved in exocytosis and endocytosis at the neuronal synapse, we still have not achieved the comprehensive understanding of these processes or the generation of synaptic vesicles. In the laboratory of Synaptic Vesicle Dynamics, we are tackling various aspects of processes that regulate synaptic vesicle formation using mouse and mammalian cells as model systems, the latest imaging, electrophysiological and cell biological techniques. Specifically, we are studying some of the key proteins (endophilin-A, synaptojanin-1, auxilin) involved in clathrin-mediated endocytosis, one of the main pathways of synaptic vesicle recycling that depends on clathrin coat formation and dissociation.

In a distinct but related line of work, we are also exploring the signaling processes that arise from altered synaptic vesicle recycling and neurotransmission, and lead to neurodegeneration (the progressive loss of structure and/or function of neuronal cells) and neurodegenerative diseases. Neurodegenerative diseases impose a significant burden on patients, families and societies, and will become of even greater concern as life expectancy increases and the world population continues to age. For decades, defective neurotransmission has been implicated in neurodegeneration and in neurodegenerative diseases, yet the molecular mechanisms remain unclear.
Recently, mutations in two clathrin-coated uncoating factors, auxilin and synaptojanin-1, have been found to cause early-onset Parkinsonism. Auxilin is recruited to clathrin coats due to the action of synaptojanin-1, which is itself brought to clathrin-coated pits by the key endocytic adaptor, endophilin-A. Remarkably, endophilin-A is directly linked to Parkinson’s disease and neurodegeneration: it is up-regulated in the cortex of PD patients and it interacts with two hallmark PD proteins: the E3 ubiquitin ligase Parkin and the leucine-rich-repeat-kinase 2 (LRRK2), the most commonly disrupted gene in familial PD. In addition, it has also recently been reported that a partial loss of endophilin in mice results in neurodegeneration, ataxia and early lethality, and that the levels of Parkin are increased in endophilin mutants.
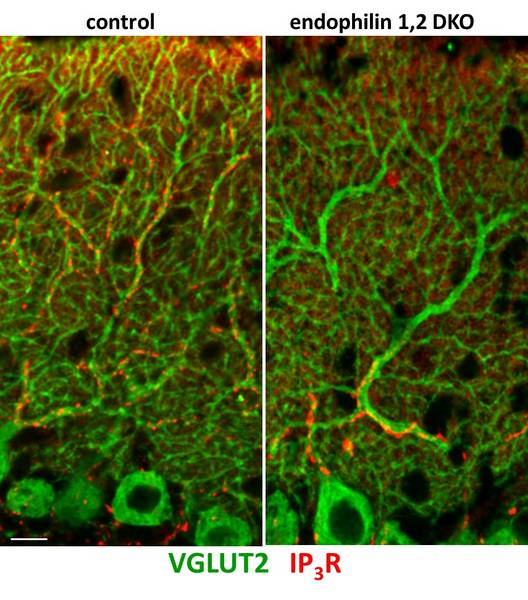
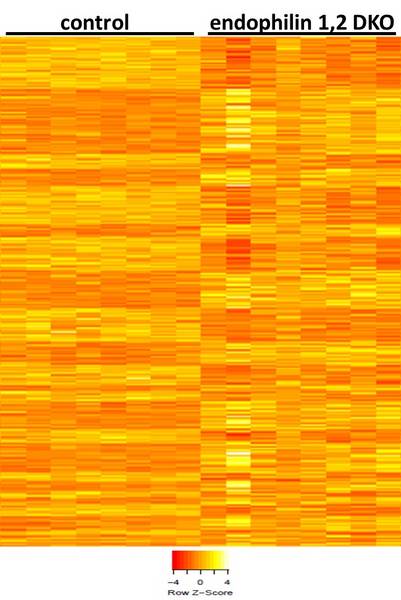
We are now employing high-throughput RNA sequencing on mice that display defective endocytosis and neurodegeneration to explore the molecular mechanisms of neurodegeneration resulting from endocytic defects. We are generating whole transcriptome profiles for these mutant mice and applying multi-dimensional genomic analysis to look for higher levels of regulation (e.g., signaling pathways and networks). We believe that these and aforementioned experimental approaches are synergistic, and will provide new insights into the mechanisms of neurodegeneration that result from defective neurotransmission. Given the central role of synaptic transmission in nervous system function, these topics are relevant for the understanding and potential treatment of severe neurodegenerative and neurological diseases.
Our work is supported by the German Research Foundation (DFG; Emmy Noether award), Alexander von Humboldt (AvH) Foundation, Schram Foundation, Engelhorn Foundation, SB889 and SFB1190.







